Embedded Software
Firmware development techniques for powering connected medical devices with safety, efficiency, and regulatory readiness.
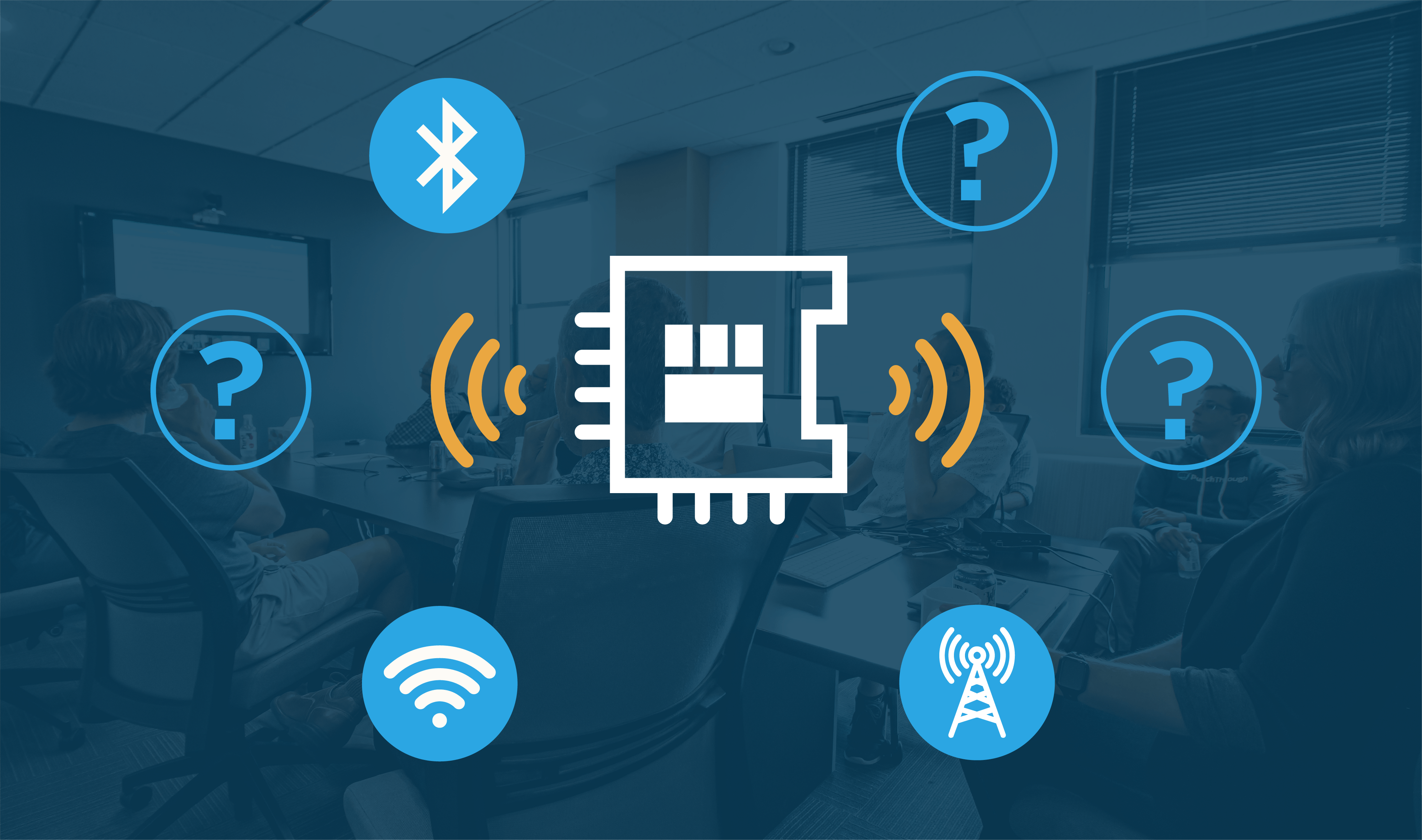
BLE Module vs Chip-Down: What is Best for Your Design?
August 24, 2022 • Matt Dunham

How to Choose the Right Hardware Platform for Connected Products
Choosing a hardware platform is one of the most critical decisions in connected product development.This guide breaks down the trade-offs that matter most and how to navigate them with confidence.

Why Early Firmware Planning Matters More Than You Think
Poor planning in embedded firmware development can lead to costly delays, rework, and regulatory risk—especially in connected medical devices. This article breaks down four early decisions that have long-term impact: chip selection, manufacturing alignment, testing strategy, and security.

Building a Risk-Aligned Testing Strategy for Your Embedded Medical Device
Build a compliant embedded testing strategy that aligns with real-world risk, supports IEC 62304 and ISO 14971, and helps your connected medical device meet regulatory and safety expectations.

How to Choose the Right Testing Strategy for Embedded Medical Devices
A smart embedded medical device testing strategy starts early—well before verification. This article breaks down key testing types, why classification alone isn’t enough, and how to align your test plan with real-world risk.

Avoid Firmware Failures with a System-Level Approach to Connected Medical Devices
Firmware doesn’t operate in isolation, here we explore how a system-level approach helps prevent integration failures, from planning through delivery.

Preventing Integration Failures with Early Firmware Design
Design firmware with integration in mind from day one to uncover mismatches early, cut costly surprises, and deliver reliable, scalable connected devices that meet real world conditions and tight timelines.
Dive into our complete collection of articles, guides, and resources.